Tryptamines

Tryptamine is a monoamine alkaloid. It contains an indole ring structure, and is structurally similar to the amino acid tryptophan, from which the name derives. Tryptamine is found in trace amounts in the brains of mammals and is hypothesized to play a role as a neuromodulator or neurotransmitter. Similar to other trace amines, tryptamine binds to human trace amine-associated receptor 1 (TAAR1) as an agonist.
Tryptamine is the common functional group in a set of compounds termed collectively substituted tryptamines. This set includes many biologically active compounds, including neurotransmitters and psychedelic drugs.
The concentration of tryptamine in rat brains is about 3.5 pmol/g.
Tryptamine is the common functional group in a set of compounds collectively termed "substituted tryptamines." Pharmacologically, substituted tryptamines are best known for being able to produce psychedelic and entheogenic effects.
An investigation of dozens of psychoactive tryptamine compounds was published by Ann and Alexander Shulgin under the title TiHKAL ("Tryptamines I Have Known and Loved").
e metabolite that is mainly responsible for the analgesic effect of tramadol, O-DSMT is significantly more potent by weight than its parent compound.
2-MeO-TMT (5-MeO-TMT) [OUT OF STOCK]
PLEASE READ BEFORE YOU MAKE AN ORDER!!Will be shipped out from our Canada warehouse. Shipped without..
$60.00
4-ACO-DET
4-Acetoxy-DET (4-Acetoxy-N,N-diethyltryptamine), also known as ethacetin, ethylacybin or 4-AcO-DET i..
$270.00
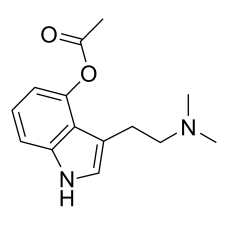
4-ACO-DMT (finished product)
4-aco-dmt is illegal in China now. You can order 4-aco-det and 4-ho-met.This l..
$21,000.00
4-ACO-EPT [OUT OF STOCK]
4-AcO-EPT Fumarate4-Acetoxy-N-Ethyl-N-propyltryptamine FumarateMolecular Formula: C19H23N2O2Molecula..
$160.00
4-AcO-MALT
4-Acetoxy-MALT (or 4-AcO-MALT) is a psychedelic tryptamine thought to be a serotonergic psychedelic ..
$140.00
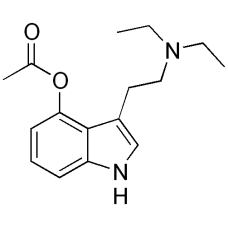
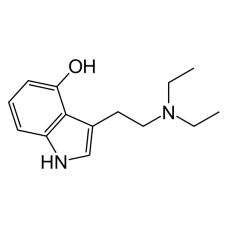
4-HO-DET
4-HO-DET is a synthetic tryptamine. Very little data exists about the pharmacological properties, me..
$185.00
4-HO-McPT
4-HO-McPT is an synthetic substituted aromatic compound and a lesser-known psychedelic tryptamine. I..
$150.00
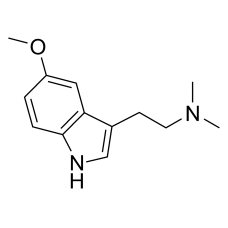
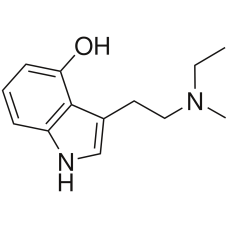
4-HO-MET
4-HO-MET (4-hydroxy-N-methyl-N-ethyl tryptamine, or metocin, methylcybin, Colour), is a lesser-known..
$270.00
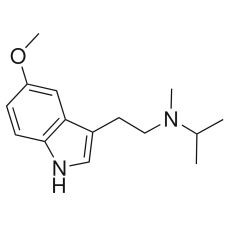
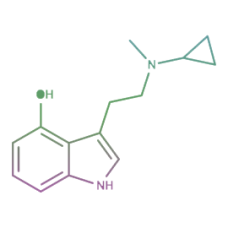
4-HO-MiPT
4-HO-MiPT (miprocin, 4-hydroxy-N-methyl-N-isopropyltryptamine) is a synthetic substituted aromatic c..
$180.00
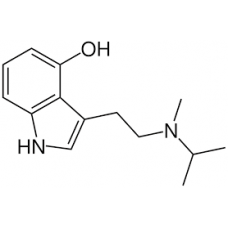
4-MeO-MiPT [OUT OF STOCK]
4-MeO-MiPT is a synthetic tryptamine. Very little data exists about the pharmacological properties, ..
$200.00
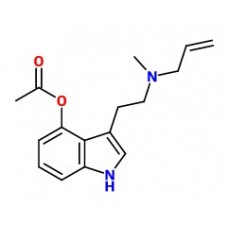
4-PRO-DMT
4-PRO-DMT .FUMARATE:Synonyms: O-PropionylpsilocinIUPAC NAME: [3-[2-(dimethylamino)ethyl]-1H-indol-4-..
$320.00
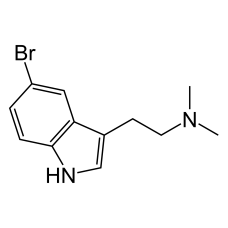
5-Bromo-DMT
5-Bromo-DMT (5-bromo-N,N-dimethyltryptamine) is a psychedelic brominated indole ..
$200.00
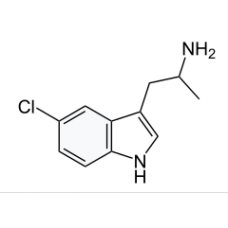
5-CHLORO-ΑMT
5-Chloro-α-methyltryptamine (5-Chloro-αMT), also known as PAL-542, is a tryptamine derivative relate..
$90.00
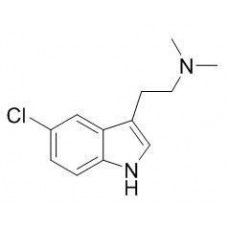
5-CL-DMT
5-CL-DMT (5-cl-N,N-dimethyltryptamine) is a psychedelic chlorinated indole alkaloid found in the spo..
$95.00
5-MeO-AI (MEAI)
5-MeO-AI / MEAI (HYDROCHLORIDE)Special tryptamine.MEAI (5-methoxy-2-aminoindane or 5-..
$60.00
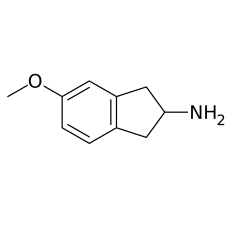
5-MeO-AMT (Alpha-O) [OUT OF STOCK]
PLEASE READ BEFORE YOU MAKE AN ORDER!!5-MeO-aMT or 5-methoxy-α-methyltryptamine, α,O-Dimethylseroton..
$90.00
5-MeO-DMT
5-MeO-DMT (5-methoxy-N,N-dimethyltryptamine) belongs to the tryptamine class and is found in a ..
$160.00
5-MeO-DMT [For Australia Customers]
This is 5-MeO-DMT order for our customers from Australia only. If you from another country, please m..
$170.00
5-MeO-MiPT
5-MeO-MiPT is a psychedelic and hallucinogenic drug, used by some as an entheogen. It has structural..
$160.00
5-MeO-MiPT [USA to USA]
5-MeO-MiPT is a psychedelic and hallucinogenic drug, used by some as an entheogen. It has structural..
$290.00

![2-MeO-TMT (5-MeO-TMT) [OUT OF STOCK] 2-MeO-TMT (5-MeO-TMT) [OUT OF STOCK]](https://thetgcrc.com/image/cache/catalog/2-meo-tmt-228x228.png)
![4-ACO-EPT [OUT OF STOCK] 4-ACO-EPT [OUT OF STOCK]](https://thetgcrc.com/image/cache/catalog/4-aco-ept-228x228.PNG)
![4-MeO-MiPT [OUT OF STOCK] 4-MeO-MiPT [OUT OF STOCK]](https://thetgcrc.com/image/cache/catalog/4-MeO-MiPT-228x228.png)
![5-MeO-AMT (Alpha-O) [OUT OF STOCK] 5-MeO-AMT (Alpha-O) [OUT OF STOCK]](https://thetgcrc.com/image/cache/catalog/5-meo-amt-228x228.png)